In the middle of 2020, half a year after COVID-19 changed the world as we knew it and we collectively entered a global pandemic, the STOPCOVID project at the Translational Healthcare Technologies group began recruiting participants for a drug trial called Define.
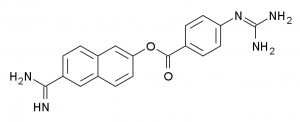
Nafamostat Mesylate
Define is a randomised controlled trial designed to provide safety and pharmacokinetic (PK)/ pharmacodynamic information for re-purposed or experimental medications that can be beneficial in COVID-19. Intravenous nafamostat was one of the first potential target drugs to be included in the trial.
Nafamostat is a synthetic serine protease inhibitor that directly inhibits the TMPRSS2 dependent stage of host cell entry. It has been used to treat disseminated intravascular coagulation, acute pancreatitis, and as an anticoagulant since the 1980s. Based on its significant antiviral properties and the prominent inhibition of thrombotic pathways and endothelial inflammation in COVID-19 – nafamostat was identified as a potential treatment.
Participants were recruited between September 2020 and February 2021 from two teaching hospitals in Edinburgh: The Royal Infirmary and The Western General Hospital. With 299 patients being screened for eligibility, resulting in only 66 being eligible, this translated into patients being split in a 1:1:1 ratio between, Nafamostat, another candidate drug and standard of care. There were 21 patients in the nafamostat group. The primary endpoint was to evaluate the safety and tolerability of nafamosat.
Whilst many repurposed drugs have progressed rapidly to large Phase 2 and 3 trials in COVID-19, Define assessed the safety and in-depth pharmacokinetics and pharmacodynamic (PK/PD) profile of nafamostat. The use of intravenous Nafamostat was not well tolerated in patients hospitalised with COVID-19, with more adverse effects appearing. We did not see any evidence of anti-inflammatory, anticoagulant or antiviral activity with intravenous nafamostat.
However, given its promising mechanism of action, nafamostat is still a drug of interest and calls for further evaluation, potentially via alternative routes.

